
Bangalore, India
Nearly 20 years’ comprehensive experience with success in driving Process Development in Clinical Trials and Medical Affairs and Clinical Development support for pharmaceutical products
International experience in medical affairs and clinical trials in USA and India.
Medical Affairs | Regulatory and Clinical Development | Clinical Research – Project and Line Management|Talent and Training Management | Medico Marketing | New Product Development | Medical Affairs|Clinical Development | Safety Technical Reporting | Documentation | Protocol Designing | Feasibility Studies
◉ Highly skilled in shaping technology demand, collaborating with internal stakeholders and making appropriate recommendations that positively impact business operations, procedures & processes
◉ Comprehensive experience in working with Global Pharmaceutical, Nutrition and Consumer care segments entailing product development across India, UAE, Europe, South East Asia, North & South American Market
◉ Skilled in defining R&D stability strategy, driving R&D stability programs, providing shelf-life assignments and preparing technical reports & regulatory documentation associated with all R&D product development initiatives
◉ Possess strong knowledge of Anti-infective, Cardiac and Neurology Products, Gastroenterology, Oncology,Nutrition & Metabolic Disorders like Diabetes/ Obesity, Clinical research, Safety Reporting, Medico-Marketing & Regulatory Affairs
◉ Strong project management skills, coupled with successful track record bringing products to domestic markets, on time and within budgeted clinical outcomes.
1992
GULBARGA UNIVERSITY
MBBS
1999
BANGALORE UNIVERSITY
MD
1999
NATIONAL BOARDS -NEW DELHI
DNB
◉ ECFMG Certification from Educational Commission for Foreign Medical Graduates
◉ Federal Licensing exams, State of PA (USA) from State Licensure Examinations
07/2018 – present
◉ Independent medical consultant to key areas of functioning like product development, assessment of markets based on the therapeutic areas, formulation clinical testing, early phase product safety testing and efficacy trials for pharmaceutical, nutraceuticals and consumer care products.
◉ Assignments with several Indian Manufacturers and Pharma. Cosmetic and food companies for clinical development of products.
◉ Working on patent protected ideas in trials to file final patent applications and for later application in Skin and Hair products.
◉ Robust clinical practice has physician at my current place of residence and Aster RV hospitals. Manage many patients a day in all areas of skin, hair and nail disorders, immunology, sexual medicine & aesthetics.
01/2013 – 05/2018
◉ Aligning Drug Discovery, Pharmaceutical & Consumer products ideation with pre-clinical & clinical feasibility to pave way for new product development.
◉ Medico marketing support with CME support, Clinical development, medical support to project management, medical safety and staff training.
◉ Assessing requirement and feasibility of research projects involving interface with top management; communicating with the consumers to identify their current and future requirements.
◉ Creating lab strategy and proposing disruptive innovations for the product category; designing robust platforms that can be cascaded into winning formulas matching the defined performance objectives.
◉ Developing descriptions for the examinations, procedures, research methods and services designed for the formulation substances; sanctioning QA specifications for the formulation substances.
◉ Drafting scientific communication describing the technological positioning and performance of the products.
◉ Providing troubleshooting support in case of complex problems to ensure that robust, stable & scalable formulation is developed.
◉ Spearheading phase II-IV trials on products both Pharmaceutical, Nutritional and Consumer Products covering multiple therapeutic areas; adhering establish regulatory compliance for Indian and Global Registrations and Licenses
◉ Operating as medical resource to the company as a whole and particularly to the clinical research department (protocol and CRF writing, adverse events, discussions with investigators, internal meetings)Highlight
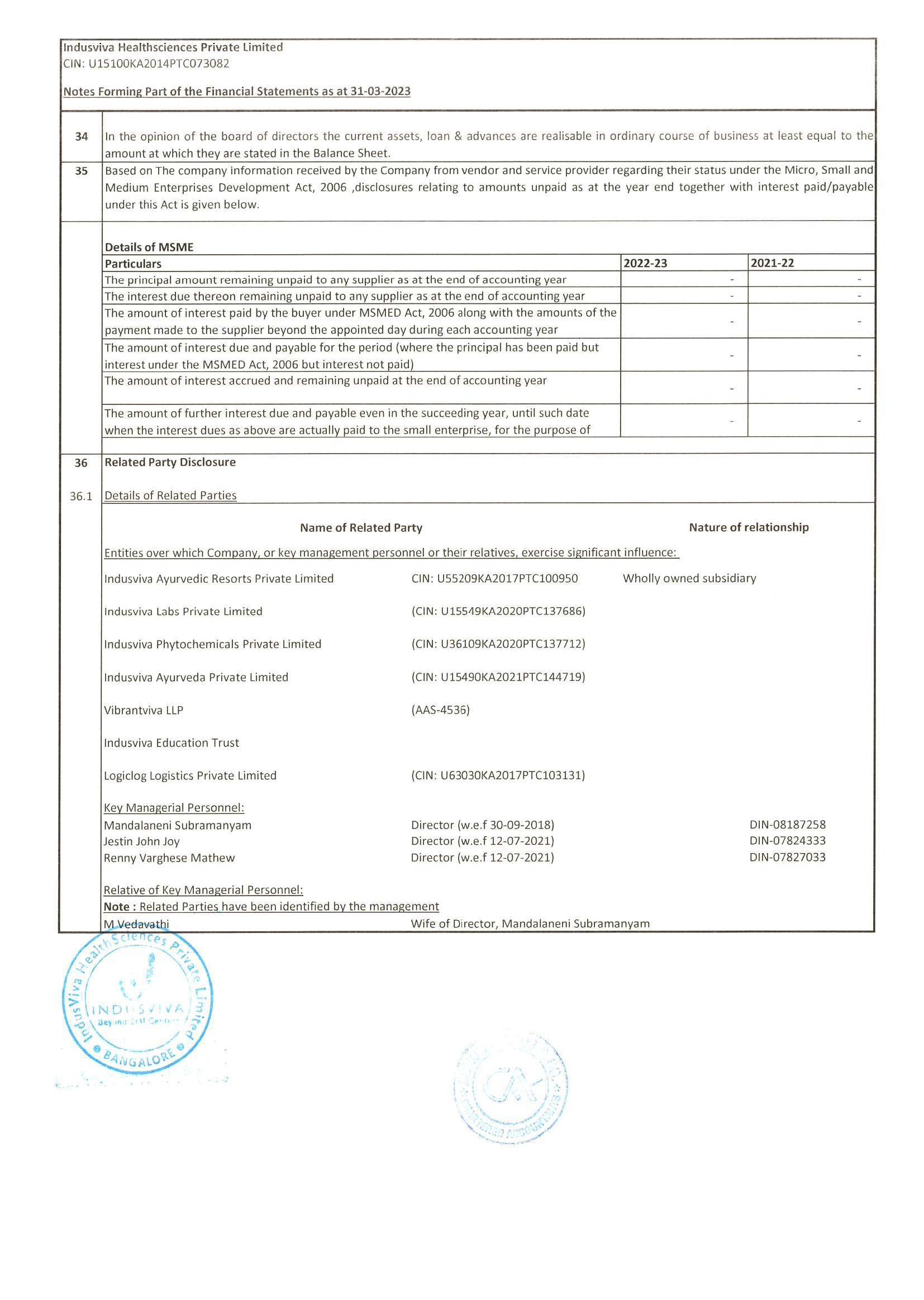
◉ Recognized as Company Expert in nominated therapeutic areas & provided input into global product development teams.
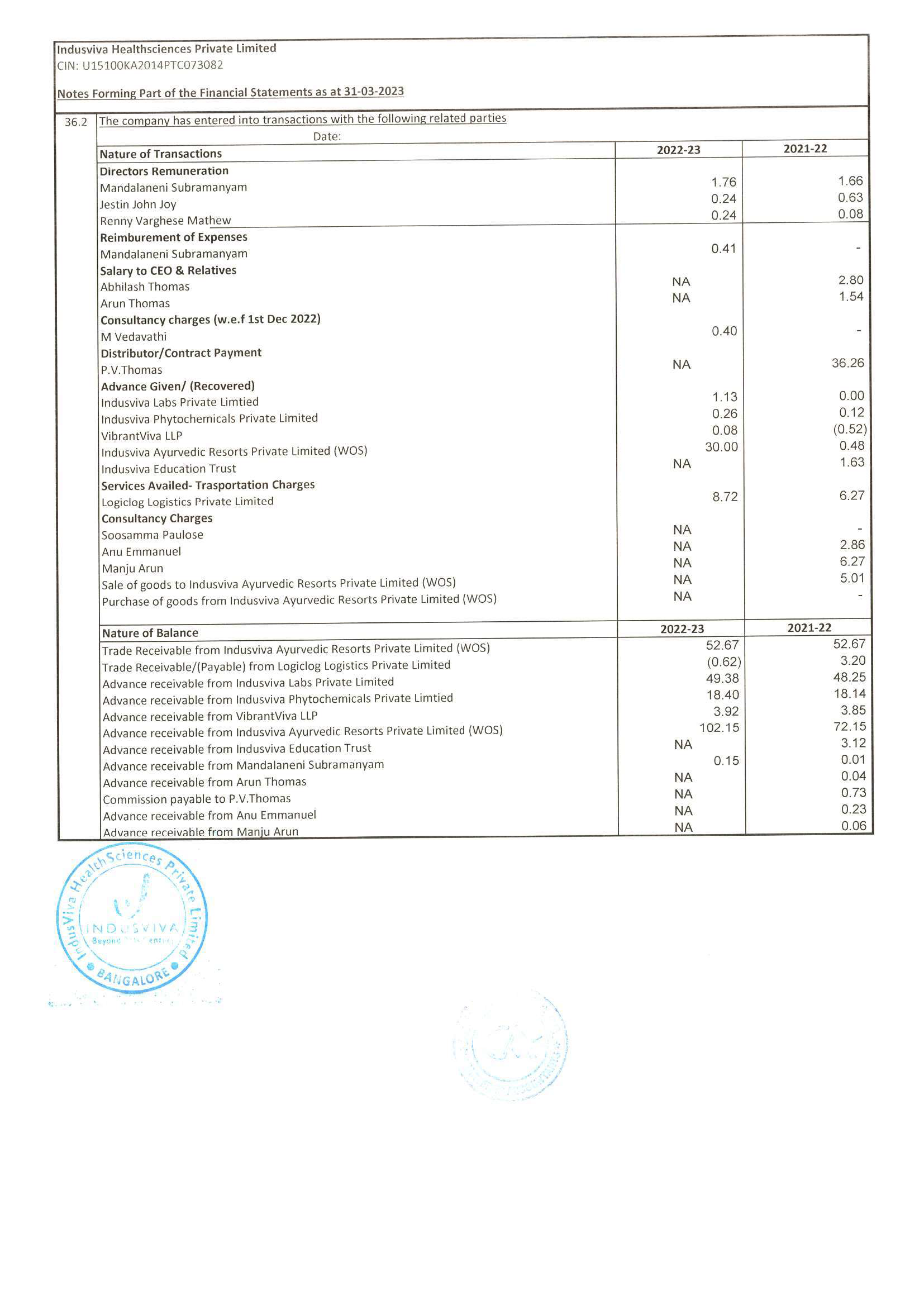
◉ Represented the company via presentations to groups of experts, societies, regulatory bodies and at international meetings; provided informative responses to scientific queries from external customers and internal partners.
◉ New Product Development: Development of Youth Eternity- Age defying range of products: Successfully launched age defying day, night and under eye creams for Himalaya for which new in vitro assays were developed to develop novel claims on the patented active showing good anti-oxidant properties
◉ Planned a bridging Clinical Study on important natural pharmaceutical medications Septillion and bresol through a pre-clinical concept of rebalancing Th1 and Th2 subsets of immunological cells and the study is progressing well in establishing an important immunological rebalance in infections and allergic disorders.
◉ Supported the development of Novel In-vitro assays for Derma care products and its development: New in vitro models were developed to screen the potential candidates for skin ailments like Psoriasis, Eczema, Atopic Dermatitis, and Xerosis; shortlisted formulations are under clinical trials, one formulation based on Skin Hydration Properties of Rice Ceramide for effective management of xerosis is being launched in 2016
◉ New claim substantiation of existing products: Led cross-functional teams in development of new claim
07/2008 – 12/2012
◉ Collaborated with sites for completion & collection of Investigator/Site essential regulatory documents such as CVs, Ethics Committee (and/or Scientific Committee) Membership List, Ethics Committee (and/or Scientific Committee) Approval, Laboratory related documents.
◉ Conducted medical and therapeutic sessions with CRA’s, undertook various projects, worked on company systems like Interix Finance, Oracle PeopleSoft, Medi-data and inform 4.0 e-CRF databases, Interix and Clinical informatics platforms, 200 monitoring visits in different therapy areas with focus on timely and quality data assessment at clinical site.
◉ Medical monitoring role on safety issues through use of Infusoria and remote risk monitoring.
◉ Participated in MPRMs and kept track of CRA’s progress in the project and utilization.
◉ Undertook various transitions, collaborated with Site Managers (CRA’s) and conducted discussion with CTL’s/PM on course of action.
◉ Allocated resources to projects and ensured the quality of assigned staff’s clinical work by regular evaluations.
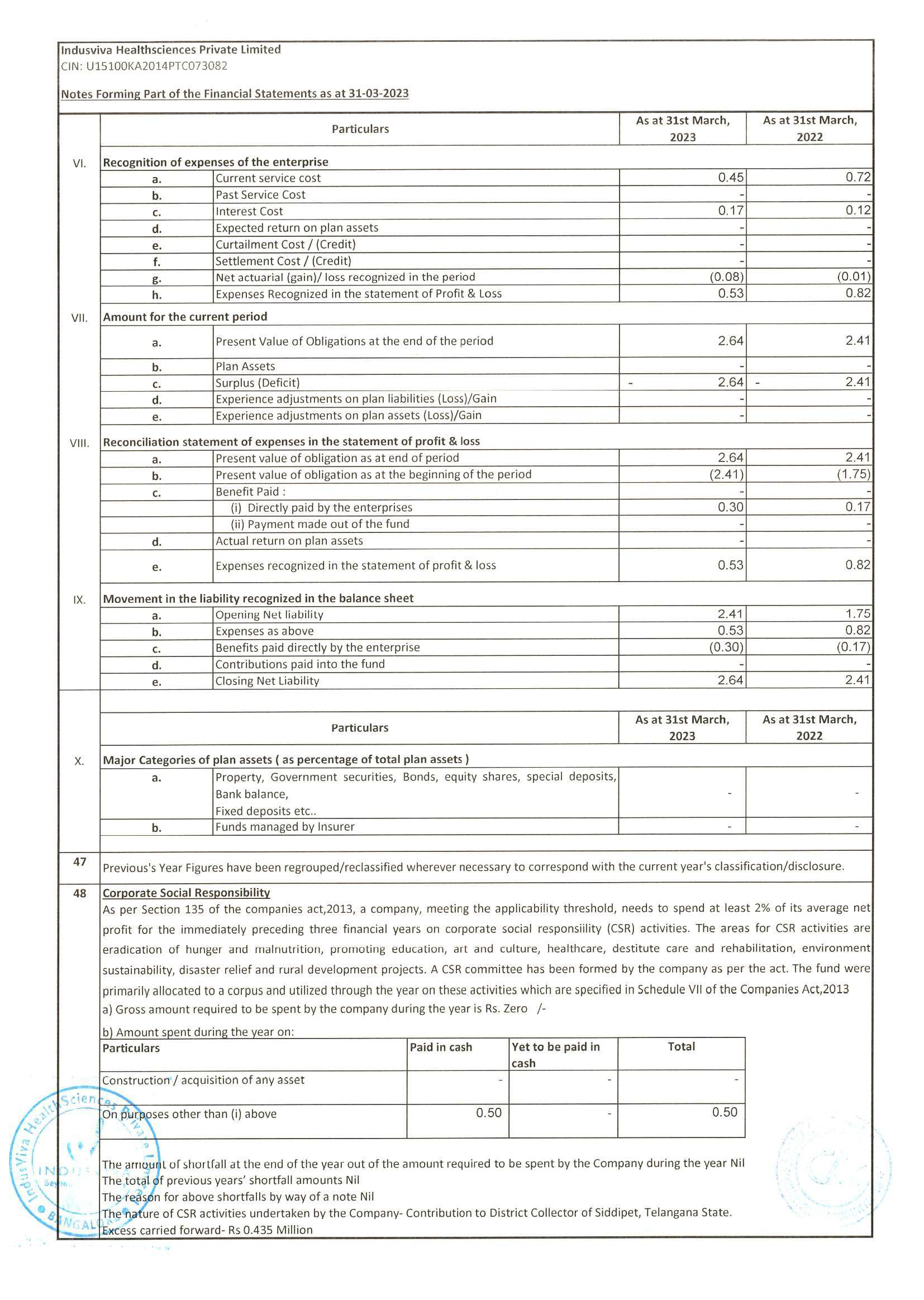
◉ Attained knowledge of ICH-GCP, FDA and EMEA regulations Highlights
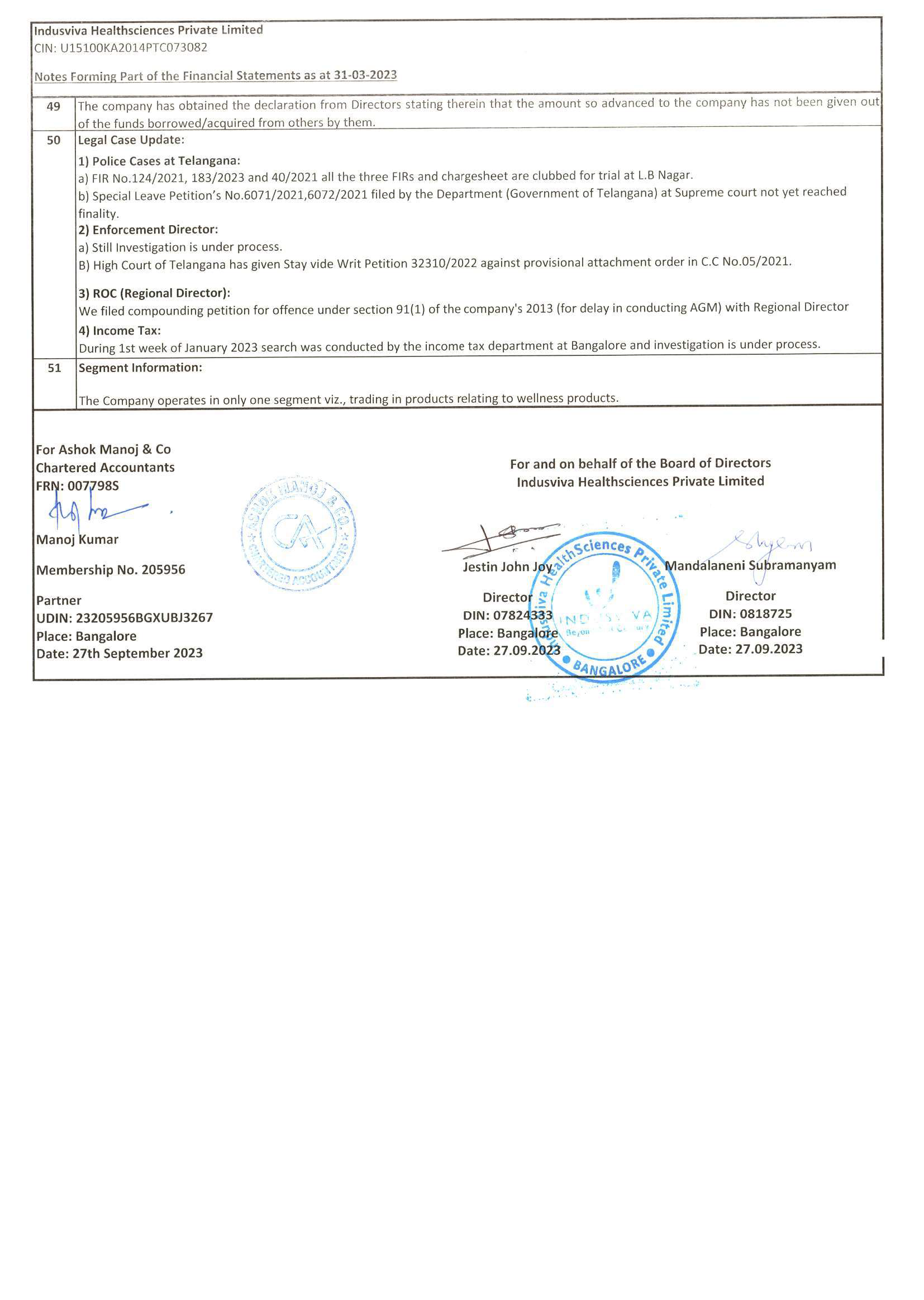
◉ Spearheaded key projects with Line management of an average of 15-20 CRA’s at an operational level that helped leverage people skills, mentoring, ensuring proper project deliverable and resource utilization
◉ Led the development and delivery of clinical operational skills workshops to new staff; led the successful sign-off.
02/2006 – 04/2008
◉ Delivered CME activities for therapy management; prepared brochures and visual materials for new drug launches.
◉ Prepared study protocols and CRF’s for various Phase III & Phase IV clinical trials
◉ Planned medico-marketing communications and training programs to marketing executives on the basics of medicine, pharmacology and related drug therapies.
◉ Collaborated with healthcare providers for introduction of new therapies and planning approval of drug extensions, new launches; prepared brochures and visual text materials for new drug launches.
07/2004 – 01/2006
◉ Organized CME activities for therapy management for Infectious diseases, Cardiac, Nutrition and gastro range of products.
◉ Delivered medico-marketing communications and training programs to marketing executives on the basics of medicine, pharmacology and related drug therapies.
◉ Training programs to marketing executives on basics of medicine, pharmacology and related drug therapies
◉ Interacted with health care providers for new therapy initiatives.
12/2001 – 04/2004
◉ Initiated Phase III/IV clinical trial projects in Dermatological, Orthopaedic, Psychiatry and Oncology therapy areas.
◉ Provided medical support to Consumer products like Recital, Volini and other range of products.
◉ Training programs to marketing executives on basics of medicine, pharmacology and related drug therapies.
◉ Interacted with health care providers for new therapy initiatives.
12/1999 – 12/2001
◉ Organized and delivered CME activities for therapy management for Infectious diseases, Cardiac, Nutrition and Gastro
◉ Prepared brochures and visual materials for new drug launches.
◉ Prepared study protocols and CRFs for various Phase III and Phase IV clinical trials
◉ Interacted with health care providers for introduction of new therapies and planning approval of drug extensions, new launches.
04/1993 – 08/1995
Hands-on training on both simple and sophisticated techniques like resting and post drug BMR estimation, Insulin-glucose clamp, whole body DEXA scan, radioisotope and lab techniques for blood glucose estimations besides managing the medical aspects of subjects on the trial.
01/1991 – 03/1993
TRAINING
◉ ICH-GCP
◉ 21 CFR Part 11
◉ Indian Regulatory Requirements
◉ CRA (Informed Consent Forms, Safety Reporting, Source Data Verification, Investigational Product, Filing)
◉ Advanced Monitoring Skills
◉ Clinical Informatics
◉ SAP –Project Lifecycle Management
◉ Project Management Training
◉ Presentation Skills
◉ Systems training (clinical, Medidata, InForm, Intraxx and Project Finance QWIP and Oracle PeopleSoft, PLM)
◉ Site Start up training and Line Management training
◉ Soft Skills Sessions – (Handling Conflicts, Managing Difficult People, Stress and Time Management, Communication Skills)
PUBLICATIONS
◉ Hyperreflexia in leprosy. Indian J Lerp. 1999 Jul-Sep; 71(3):342-3
◉ Leprosy presenting as macrocheilia. Indian J Lepr. 1999 Jul-Sep; 71(3):341
◉ Study on clarithromycin gel for the first time in Indian Acne patients Indian medical Gazette –Dec2003
◉ Study on Valdecoxib in Indian Patients with OA and RA Indian Medical Gazette- Feb 2004 Protective effects of Triphala on dermal fibroblasts and Human keratinocytes PLos One 11(1) e0145921.IF:3.7
◉ Anti-inflammatory and skin protective properties of virgin coconut oil in vitro Journal of Cosmetic Science. Communicated-2016.
◉ Imiquimod-induced Psoriasis-like inflammation in differentiated Human keratinocytes: A new model for screening anti-psoriatic drugs and its evaluation using curcumin. Journal of Investigative dermatology European Journal of Pharmacology
PROFESSIONAL AFFILIATIONS
◉ Member – American Medical Association 1995
◉ Life Member- IADVL Since 2005
◉ Joint Secretary –Bangalore Dermatological Society – April 2015
You Cannot Copy Content











| 1028 | 1030 | 1227 |
| 4719 | 4723 | 5176 |
| 15736 | 15843 | 21819 |
| 38221 | 38587 | 38678 |
| 39769 | 39818 | 41747 |
| 43494 | 43574 | 50044 |
| 50570 | 53142 | 54945 |
| 59447 | 65762 | 67434 |
| 75673 | 88452 | 92594 |
| 102483 | 107480 | 133350 |
| 136546 | 139347 | 142681 |
| 146449 | 146885 | 150288 |
| 151677 | 159459 | 200147 |
| 209295 | 216865 | 227597 |
| 261587 | 392845 | 403012 |
| 501161 | 571545 | 581896 |
| 585835 | 643456 | 95538 |
| 180227 | 67756 | 31683 |
| 77753 | 31209 | 369915 |
| 39952 | 153538 | 5192 |
| 391744 | 26413 | 1328 |
| 2518 | 92395 | 59235 |
| 1005 | 402428 | 51262 |























































![]()























































[gtranslate]
[wprevpro_usetemplate tid=”1″]
